When we observe our surrounding, we find that every object present around us is composed of much smaller units that act as the building blocks of that object. Whether it is solid, liquid or gas, it is composed of much smaller units which are arranged in a certain manner to obtain that substance. This small unit is called molecule.
A molecule is an electrically neutral group of two or more atoms chemically bonded together. The forces which hold the atoms together in a molecule are called covalent bonds. Thus, a combination of atoms is called a molecule.
Atoms of many elements are very reactive and cannot exist in the free state (as single atom). But molecules can exist in free state because they are very stable. So, we can also define molecule as the smallest particle of a substance (element or compound) which has the properties of that substance and can exist in the free state.
Molecules of element –
The molecule of an element contains two or more similar atoms chemically combined together.
For example – molecule of hydrogen gas (H2) contains 2 atoms of H, molecule of nitrogen gas (N2) contains 2 atoms of N, molecule of oxygen (O2) contains 2 atoms of O. Similarly, bromine, iodine, chlorine, all of these consists of 2 atoms of each of these elements chemically bonded together to form a molecule of these gases. One molecule of ozone consists of 3 atoms of oxygen bonded together. A molecule of phosphorous consists of 4 atoms of phosphorous and a molecule of Sulphur consists of 8 atoms of Sulphur chemically bonded together. Apart from these elements, the noble gases such as helium, neon, krypton, argon etc. exist as single atoms.
The number of atoms present in one molecule of an element is called its atomicity. Some of the molecules and their atomicity are given below –
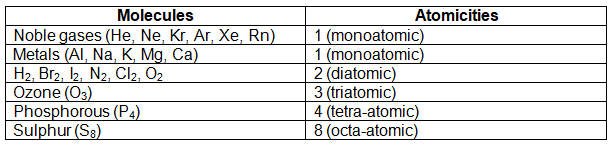
Molecules of compounds –
The molecule of compound contains two or more different types of atoms chemically combined together. For example – hydrogen chloride is a compound. The molecule of hydrogen chloride (HCl) contains two different types of atoms: hydrogen atom (H) and chlorine atom (Cl).
A compound which consists of molecules is called molecular compound. A chemical formula represents the composition of a molecule of the substance in terms of the symbols of the elements present in the molecule. A chemical formula is also known as a molecular formula.
The chemical formula of an element is a statement of the composition of its molecule in which symbol tells us the element and the subscript tells us how many atoms are present in one molecule. For example – the formula of hydrogen gas is H2. The figure 2 in the formula indicates that one molecule of hydrogen element contains 2 atoms of hydrogen. Similarly, formula of oxygen is O2, formula of nitrogen is N2, and formula of chlorine is Cl2. The inert gases such as helium, neon, argon are monoatomic, so their symbols and formula are just the same.
The chemical formula of a compound is a statement of the composition of its molecule in which symbol tells us the element and the subscript tells us how many atoms are present in one molecule of the compound. Water is a compound whose molecular formula is H2O so, it contains 2 atoms of Hydrogen and 1 atom of oxygen chemically bonded together. Also, 2 H2O means 2 molecules of water. Number 2 multiplies with the complete formula and not just the first element. Therefore, 2H2O contains 4 atoms of hydrogen and two atoms of oxygen.
Molecular mass –
Just as an atom has atomic mass, in the same way a molecule has molecular mass. The molecular mass of a substance is the relative mass of its molecule as compared with the mass of a carbon-12 atom taken as 12 units. The molecular mass is expressed in atomic mass unit (u).
If the molecular formula of a substance is known, its molecular mass can be calculated, because the molecular mass is equal to sum of the atomic masses of all the atoms present in one molecule of the substance. For example, one molecule of water (H2O) contains 2 atoms of hydrogen and 1 atom of oxygen. So, the molecular mass of water can be calculated as –
Mass of H atom = 1 u
Mass of 2 H atoms = 2 u
Mass of O atom = 16 u
Now, molecular mass of H2O = mass of 2 H atoms + mass of O atom
= (2 +16) u = 18 u
In case of ionic compounds like sodium chloride which consists of ions and not molecules the term formula mass is used in place of molecular mass.
NCERT textbook Solutions Page 39
Question 3:-
What is meant by the term chemical formula?
Answer :-
The chemical formula of a compound represents the composition of a molecule of the compound in terms of the symbols of the elements present in it. For example, the chemical formula of water H2O tells us that one molecule of water is made up of 2 hydrogen atoms and one oxygen atom.
Page 40
Question 1:-
Calculate the molecular masses of H2,O2, Cl2, CO2, CH4, C2H6, C2H4, NH3, CH3OH. (Atomic masses: H=1; O=16; Cl=35.5; C=12; N=14)
Answer:-
- Molecular mass of H2= 2 x mass of H atom = 2 u
- Molecular mass of O2= 2 x mass of O atom = 32 u
- Molecular mass ofCl2 = 2 x mass of Cl atom = 2 x 35.5 = 71 u
- Molecular mass ofCO2 = mass of C + 2 x mass of O = 44 u
- Molecular mass ofCH4= mass of C + 4 x mass of H = 16 u
- Molecular mass ofC2H6= 2 x mass of C + 6 x mass of H = 30 u
- Molecular mass ofC2H4 = 2 x mass of C + 4 x mass of H = 28 u
- Molecular mass ofNH3 = mass of N + 3 x mass of H = 17 u
- Molecular mass ofCH3OH = mass of C + 4 x mass of H + mass of O = 32 u
Question 2:-
Calculate the formula unit masses of ZnO, Na2O, K2CO3. (Given: Atomic masses of Zn=65u; Na=23u; K=39u; C=12u and O=16u)
Answer:-
(i) Formula mass of ZnO
= mass of Zn atom + Mass of O atom
= 65+16
= 81u
(ii) Formula mass of Na2O
= 2 x mass of Na atom + mass of O atom
= 2 x 23 + 16
= 46 +16 = 62u
(iii) Formula mass of K2CO3
= 2 x mass of K + mass of C + 3 x mass of O
= 2 x 39 + 12 + 3 x 16 = 138u
Conclusion – A molecule is an electrically neutral group of two or more atoms chemically bonded together. A chemical formula represents the composition of a molecule of the substance in terms of the symbols of the elements present in the molecule. A chemical formula is also known as a molecular formula. The molecular mass of substance is the relative mass of its molecule as compared with the mass of a carbon-12.