When a liquid remains exposed to air its volume decreases gradually. This is because of an evaporation. During this process, some of the surface liquid becomes vapor or gas, and mixes during air with the gases. Thus, it is said to evaporate when a liquid turn into vapour or gas. Evaporation is influenced by many factors.
We may describe evaporation as: evaporation is called the process of turning a liquid into vapour even below its boiling point. Liquid evaporation can occur even at room temperature but at higher temperatures it is faster. Whatever the temperature at which evaporation happens, the vaporizing latent heat must be supplied if a liquid transform into vapour.
Due to the evaporation of the water in them the wet clothes dry. And rainwater puddles driver as well, due to water evaporation. The evaporation process also reclaims common salt from seawater.
Factors affecting evaporation –
- Temperature
- Surface area
- Humidity
- Wind speed
To understand this, we will need to know each of this in detail.
Temperature –
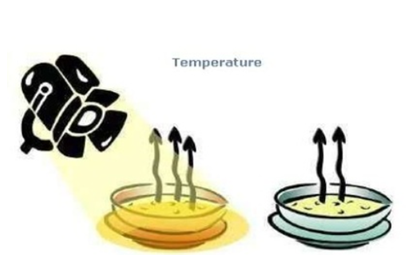
The rate of evaporation increases with the increasing temperature of the liquid. In other words, the rate at which a liquid evaporates on heating increases. Once a liquid ‘s temperature is lifted by heating it up, more liquid particles get enough kinetic energy to reach the vapor or gaseous state. That increases evaporation rate. Thus, a liquid’s evaporation rate may be increased by heating it up. At its boiling point the evaporation rate of a liquid is maximum.
Surface area of the liquid –
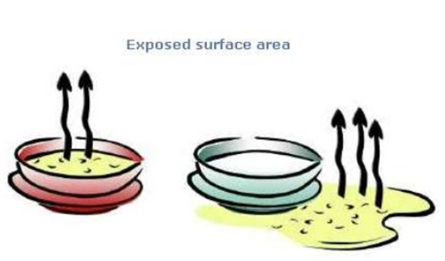
The rate of evaporation increases with the rising surface area of the liquid. And, if a liquid’s exposed air surface area is raised, the liquid’s rate of evaporation increases. For example, if the same liquid is kept in a test tube and in a china dish, then the liquid stored in the china dish will evaporate faster because more of its surface area is exposed to air. We spread the washed wet clothes in our daily lives while drying to increase their surface area for the rapid evaporation of the water present in them which leads to a faster drying out of wet clothes.
Humidity of air –
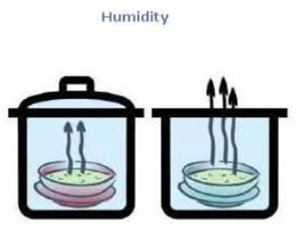
A term named humidity represents the amount of water vapor present in air. When the amount of water vapor in the air is small, the air seems dry and we say the humidity is low. On the other hand, the air appears to be damp when the amount of water vapor in the air is large and we say the humidity is high. So, air humidity tells us the degree of air dampness.
When air humidity is low, then evaporation rates are high, and water evaporates more readily.Under these circumstances, our body’s sweat evaporates readily, and we feel comfortable and cool.Under low air humidity conditions, the wet clothes dry quickly.When air humidity is high, then evaporation rate is low, and water evaporates very slowly. In the later part of summer, the humidity of air increases. People sweat a lot in such weather. But the sweat from our bodies does not evaporate readily due to high humidity of air. Such weather becomes muggy and we feel hot and uncomfortable. This type of weather is experienced during cloudy days in the rainy season and in areas close to the sea. The wet clothes take a long time to dry when the humidity of air is high.
Wind speed –
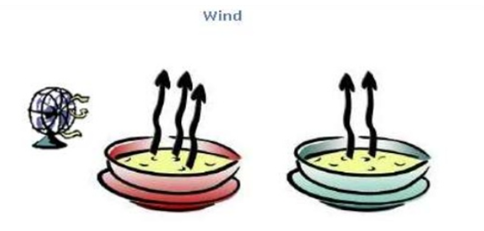
The rate of liquid evaporation increases with increased wind velocity. As the wind speed increases, the water vapor particles travel along with the wind, reducing the volume of ambient water vapour. That raises the rate of water evaporation. On a windy day, the washed wet clothes dry faster, because evaporation is faster due to high wind speed.
The factors that affect evaporation of a liquid are used in our daily lives to make use of the conditions of atmosphere. While we use evaporation for our day to day needs, keeping in mind the various factors that affect this process can increase the efficiency of the process.
Evaporation is the process of a liquid changing into vapor or gas, there are many factors affecting evaporation even beneath its boiling point. Temperature, surface area, humidity, wind speed are all different factors. The rate of evaporation increases with increasing temperature of the liquid. The rate of evaporation increases with the increasing surface area of the liquid. If the temperature in the air is low, so the rate of evaporation is high and water evaporates more readily. When air humidity is high, then the rate of evaporation is low and water is very slow to evaporate. The rate of liquid evaporation increases with increased wind velocity.