Every material present around us has some specific properties due to which we are able to differentiate between various particles. These properties are specifically about the composing units of that substance. These are called characteristics of particles of matter.
Every material is made up of small particles that combine together to produce the complete material. The characteristics of these particles are :-
- The particles of matter are very, very small
- The particles of matter have spaces between them
- The particles of matter are constantly moving
- The particles of matter attract each other
These were the properties of matter that were given after they were observed through certain experiments done by scientists.
So, to understand these properties we need to study the experiments through which they were derived.
The particles of matter are very, very small –
The very, very small size of particles of matter can be shown by an experiment using potassium permanganate. Potassium permanganate is a kind of matter. We take 2-3 crystals of potassium permanganate and dissolve it in 100 millilitres of water in a beaker. We will get a deep purple coloured solution of potassium permanganate in water.
Now we take 10 ml of this deep purple solution of potassium permanganate from the first beaker and mix it with 90 ml of water present in it second beaker, to dilute it. Due to this dilution, the colour of potassium permanganate solution in the second beaker becomes a bit lighter. Now we again take 10 ml of potassium permanganate solution from the second beaker and mix it with 90 ml of water present in the third beaker, to dilute it further.
The colour of solution will become still lighter. We keep on diluting the potassium permanganate solution like this a number of times. In this way, we get a very dilute solution of potassium permanganate in water but the water is still coloured.

This experiment shows that just 2 or 3 crystals of potassium permanganate cam impart colour to a large volume of water. From this observation we conclude that each potassium permanganate crystal is itself made up of millions of small particles which keep on spreading and imparting colour to more and more of water on dilution.
The particles of matter have spaces between them –
The spaces between the particles of matter can be shown by the experiment of water and sugar. We take about 100 ml of water in a beaker and mark the level of water with a marking pen. We also take 50 grams of sugar. Now, we add 50 grams of sugar to water in the beaker. Dissolve the sugar by stirring it with a glass rod. When all the sugar has dissolved, we get a sugar solution.
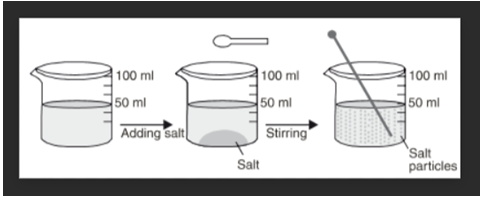
When we look at the level of water in beaker, we will find that level of sugar solution in the beaker is at the same mark where water level was initially in the beaker. Hence, the volume of water did not increase even upon adding 50 grams of sugar.
This can be explained as follows: When sugar is dissolved in water, its crystals separate into very fine particles. These particles of sugar go into the spaces between the various particles of water due to which there is no change in the volume of water on dissolving sugar in it. The fact that there is no change in volume on dissolving sugar in water tells us that there are spaces between the particles of water. And these spaces accommodate the sugar particles.
The particles of matter are constantly moving –
The best evidence that particles of matter are constantly moving comes from the studies of diffusion and Brownian motion. When we light an incense stick in one corner of the room, its fragrance spreads in the whole room quickly. This is because the burning of incense stick produces gases having pleasant smell. The particles of gases produced by burning of incense stick move rapidly in all directions, mix with the moving particles of air in the room, and reach every part of the room quickly. When the gaseous particles from the incense stick reach our nose, we can smell the fragrance.

If however, the particles of gases produced by the burning incense stick and the particles of air were not moving, then the fragrance of incense stick could not spread in the whole room quickly.
So, the observation that the fragrance of a burning incense stick spreads in the entire room very quickly tells us that the particles of matter are constantly moving.
The particles of matter attract each other –
There is some force of attraction between the particles of matter which holds them together. The force of attraction between the particles of the same substance is known as cohesion. The force of attraction is different in the particles of different kinds of matter.

If we take a piece of chalk, a cube of ice and an iron nail, and beat them with a hammer, we will find that it is very easy to breakthe piece of chalk into smaller particles, it requires more force to break a cube of ice, whereas the iron nail does not break at all even with a larger force. This shows that the force of attraction between the particles of chalk is quite weak, the force of attraction between the particles of ice is a bit stronger whereas the force of attraction between the particles of iron nail is very, very strong.
Similarly, we can move our hand through air very, very easily because the force of attraction between the particles of air is negligible. But if we try to break a piece of wood by our hand then we require a large amount of force. Hence, the force of attraction is maximum in the particles of a solid matter and minimum in the particles of gaseous matter.
Question about characteristics of particles of matter from NCERT textbook – Page 3
Question 4:-
What are the characteristics of particles of matter?
Answer 4:-
The important characteristics of the particles of matter are –
- They are very, very small.
- They have spaces between them.
- They are constantly moving.
- They attract one another.
Conclusion – The properties of matter help us to distinguish them from one another. The characteristics of particles of matter are four properties that are associated with the particles that compose matter. Particles of matter are very, very small. Particles of matter have spaces between them. Particles of matter are constantly moving. Particles of matter attract each other.