Matter is present everywhere around s and we notice that some of the matter can transform its physical state by going through some of the physical processes. So this gives rise to an interesting question – can matter change its state ?
So, it is possible to change the state of matter although it requires some unusual measures because change in state of matter is not a natural process.
We can change the physical state of matter in two ways:
- By changing the temperature
- By changing the pressure
When we say that we can change the state of matter by changing the temperature, we mean to say that the state of matter can be changed by heating it or cooling it. And when we say that we can change the state of matter by changing the pressure, we mean that the state of matter can be changed by increasing the pressure on it, or by decreasing the pressure on it.
Effect of change of temperature –
By increasing the temperature, a solid can be converted into liquid state; and a liquid can be converted into gaseous state. And by decreasing the temperature, a gas can be converted into liquid state; and a liquid can be converted into solid state.
Melting –

The process in which a solid substance changes into a liquid on heating, is called melting or fusion. So, when ice changes into water on heating, it is called melting or fusion of ice. The temperature at which a solid substance melts and changes into a liquid at atmospheric pressure, is called melting point of the substance. For example, the ice melts at a temperature of 0˚C.
Different solids have different melting points for example the melting point of ice is 0 ˚C the melting point of wax is 63 ˚C whereas the melting point of iron is 1535 ˚C. The melting point of a solid is a measure of the force of attraction between its particles (atoms or molecules).Higher the melting point of a solid substance, greater will be the force of attraction between its particles. For example, the melting point of iron metal is very high which tells us that the force of attraction between the particles of iron is very strong.
When a solid is heated sufficiently, it changes its physical state and becomes a liquid. This happens as follows: when a solid substance is heated, the heat energy makes its particles vibrate more vigorously at the melting point the particles of when a solid substance is heated the heat energy makes its particles vibrate more vigorously. At the melting point, the particles of a solid have sufficient kinetic energy to overcome the strong forces of attraction holding them in fixed positions and break to form small groups of particles and the solid melts to form a liquid.
Boiling or vaporisation –

Water normally exists in the liquid state. If we go on heating water, it ultimately starts boiling and changes rapidly into a gas called steam. In this case, the liquid water changes into a gas, so a change of state has taken place. The process in which a liquid substance changes into a gas rapidly on heating is called boiling.The temperature at which a liquid boils and changes rapidly into a gas at atmospheric pressure, is called boiling point of the liquid. Different liquids have different boiling points. For example, the boiling point of alcohol is 78 ˚C the boiling point of water is 100 ˚C, whereas the boiling point of mercury is 357 ˚C. The boiling point of a liquid is a measure of the force of attraction between its particles. Higher the boiling point of a liquid, greater will be the force of attraction between its particles.
When a liquid is heated it changes its physical state and becomes a gas. This happens as follows: when a liquid is heated, the heat energy makes its particles move even faster. At the boiling point the particles of a liquid have sufficient kinetic energy to overcome the forces of attraction holding them together and separate into individual particles. And the liquid boils to form a gas.
Condensation –

If we cool steam or water vapour by lowering its temperature coma it is converted into liquid water. In this case a gas changes into a liquid coma show a change of state has taken place. The process of changing a gas to a liquid by cooling, is called condensation. So, when steam or water vapour changes into water on cooling, it is called condensation of steam or condensation of water vapour.
A change of state takes place during condensation. This happens as follows: when a gas is cooled enough, then its particles lose so much kinetic energy that they slow down, move closer together until they start being attracted to each other, and form a liquid.
Freezing –

When water is cooled by lowering its temperature by keeping in the freezer compartment of a refrigerator, it changes into solid ice. The process of changing a liquid into a solid by cooling, is called freezing. Freezing means solidification. Freezing is the reverse of melting. So, the freezing point of a liquid is same as the melting point of its solid form.
Change of state from liquid to solid occurs during freezing. This can be explained as follows: when a liquid is cooled by lowering its temperature, its particles lose energy due to which they move slowly. If the liquid is cooled enough to its freezing point, its each particle stops moving and vibrates about a fixed position. At this stage the liquid freezes and becomes a solid.
Effect of change of pressure –
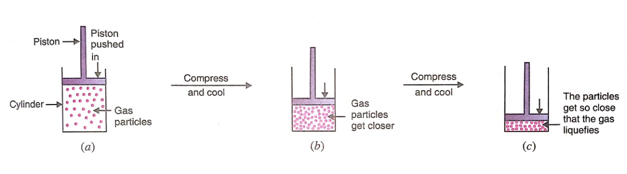
The physical state of matter can also be changed by changing the pressure. For example, gases can be changed into liquid by increasing the pressure. Andsome solids can change into gases on decreasing the pressure.
When a high pressure is applied to a gas, it gets compressed into a small volume and when we also lowest temperature, it gets liquefied. So, we can also say that gases can be liquefied by compression and cooling. This happens as follows:
There is a lot of space between the particles of a gas. We can reduce the spaces between the particles of a gas by enclosing it in a cylinder and compressing it by pushing the piston. If enough force is applied to the piston, the gas is highly compressed into a small volume. The particles of a gas get so close together that they start attracting each other sufficiently to form a liquid. And we say that the gas has liquefied. When a gas is compressed too much, then heat is produced due to compression. So, while applying pressure to liquefy gases, it is necessary to cool them to take away the heat produced during compression. Cooling lowers the temperature of compressed gas and helps in liquefying it.
Conclusion – Matter can change its state by either altering the temperature or the pressure. When we increase the temperature of a solid substance, it converts to liquid and increasing the temperature of a liquid substance, it converts into a gas. The temperature at which the solid converts into liquid it is called melting point and the temperature at which a liquid convert into gas, it is called boiling point. When we increase the pressure and decrease the temperature, the gas converts into liquid.