LAWS OF CHEMICAL COMBINATION – In chemistry every substance combines with other substances following the three laws of chemical combination. The laws of chemical combination are –
- Law of conservation of mass
- Law of constant proportions
- Law of multiple proportions
These laws of chemical combination govern all chemical reactions taking place in chemistry. These laws need to be studies in detail to avoid any confusion among each of them since they seem to be the same thing said in different words.
Law of conservation of mass –
This law of chemical combination was given after an experiment. When scientists carried out a chemical reaction in a closed container. They weighed the contents of the container before the reaction and after the reaction. Both the weights were exactly the same. Hence, Antoine Lavoisier gave the first law of chemical combination: law of conservation of mass.
Mass is neither created nor destroyed in a chemical reaction. The law of conservation of mass states that in a chemical reaction, the total mass of products is equal to the total mass of reactants. There is no change in mass during a chemical reaction.
For example – when calcium carbonate is heated, a chemical reaction takes place to form calcium oxide and carbon dioxide, it has been found that if 100 grams of calcium carbonate are decomposed completely then 56 grams of calcium oxide and 44 grams of carbon dioxide are formed.
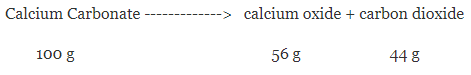
We can see that total mass of reactants = 100g
And total mass of products = (56+44) g = 100 g
Thus, change in mass during this reaction = 0
Hence, this proves that the total mass remains conserved in a chemical reaction.
Law of constant proportions –
This law of chemical combination was given by Joseph Proust in 1779. This law was given after Proust analysed the chemical compositions of a large number of compounds and came to a conclusion that the proportion of each element in a compound remains fixed. Hence, he gave the second law of chemical combination –
A chemical compound always consists of the same elements combined together in the same proportion by mass.
This law means that no matter from which source we are getting that compound from, the elements in that compound will remain in same proportions irrespective of its source. A pure chemical compound is always made up of same elements in the same percentage by mass.
For example – water is a compound which always consists of same two elements, hydrogen and oxygen which are combined together in the same proportion of 1:8 by mass (1 part by mass of hydrogen and 8 parts by mass of oxygen).
Law of multiple proportions –
This law states that whenever two elements combine to form more than one compound between them then the ratio in which the masses of one element combines with the other element will always be in whole number ratio. This law was given by John Dalton.
Questions on Laws of Chemical Combination from NCERT textbook –
Page 32 and 33
Question 1:-
In a reaction, 5.3 g of sodium carbonate reacted with 6 g of ethanoic acid. The products were 2.2 g of carbon dioxide, 0.9 g of water and 8.2 g of sodium ethanoate. Show that these observations are in agreement with the law of conservation of mass.
Answer 1:-
We need to calculate total mass of reactants and total mass of products and then show that both of them are equal.
Sodium Ethanoate + Ethanoic Acid —–> Sodium Ethanoate + Carbon Dioxide + Water
Total mass of reactants = mass of sodium ethanoate + mass of ethanoic acid
= ( 5.3 + 6 ) g = 11.3 g
Total mass of products
= mass of sodium ethanoate + mass of carbon dioxide + mass of water
= ( 8.2 + 2.2 + 0.9 ) g = 11.3 g
Since, mass of reactants = mass of products
Therefore, we can say that law of conservation of mass is valid in this case.
Question 2:-
Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas ?
Answer 2:-
Here, it is given that the ratio in which hydrogen and oxygen combine is 1:8 by mass. Hence, we can say that –
1 g of hydrogen gas requires = 8 g of oxygen gas
3 g of hydrogen gas requires = 8 x 3 g of oxygen gas = 24 g of oxygen gas
Thus, 24 grams of oxygen would be required to react completely with 3 grams of hydrogen gas.
Page 43 and 44
Question 1:-
A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Answer 1:-
Mass of boron in compound = 0.096 g
Mass of compound = 0.24 g
So, percentage of boron = (mass of boron in compound)/(mass of compound) x 100
= 0.096/0.24 x 100 = 40
Mass of oxygen in compound = 0.144 g
Mass of compound = 0.24 g
So, percentage of oxygen = (mass of oxygen in compound)/(mass of compound) x 100
= 0.144/0.24 x 100 = 60
Hence, our final answer –
Percentage of boron = 40 % and percentage of oxygen = 60 %
Question 2:-
When 3.0 g of carbon is burnt in 8.00 g of oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical composition will govern your answer?
Answer 2:-
Carbon and oxygen combine in ration of 3:8 to produce 11 g of carbon dioxide.
Therefore, with 3 g of carbon, 8 g of oxygen will react completely to produce 11 g of carbon dioxide. ( 50 – 8) g = 42 g extra oxygen will remain unreacted. Our answer is governed by law of constant proportions.
Conclusion – In chemistry, three laws of chemical combination govern all chemical reactions. These laws were given by three different scientists. The first law is law of conservation of mass which was given by Antoine Lavoisier which states that mass is neither created nor destroyed in a chemical reaction. The second law is law of constant proportions and was given by Joseph Proust which states that a chemical compound always consists of the same two elements combined together in the same proportion by mass. The third law is law of multiple proportions and was given by John Dalton and it states that whenever two elements combine then the ratio of combination of first element with second element is always in simple whole numbers.